
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Metabolic Risk/Epidemiology
- Current Status of Low-Density Lipoprotein Cholesterol Target Achievement in Patients with Type 2 Diabetes Mellitus in Korea Compared with Recent Guidelines
- Soo Jin Yun, In-Kyung Jeong, Jin-Hye Cha, Juneyoung Lee, Ho Chan Cho, Sung Hee Choi, SungWan Chun, Hyun Jeong Jeon, Ho-Cheol Kang, Sang Soo Kim, Seung-Hyun Ko, Gwanpyo Koh, Su Kyoung Kwon, Jae Hyuk Lee, Min Kyong Moon, Junghyun Noh, Cheol-Young Park, Sungrae Kim
- Diabetes Metab J. 2022;46(3):464-475. Published online March 3, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0088
- 6,946 View
- 347 Download
- 4 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We evaluated the achievement of low-density lipoprotein cholesterol (LDL-C) targets in patients with type 2 diabetes mellitus (T2DM) according to up-to-date Korean Diabetes Association (KDA), European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS), and American Diabetes Association (ADA) guidelines.
Methods
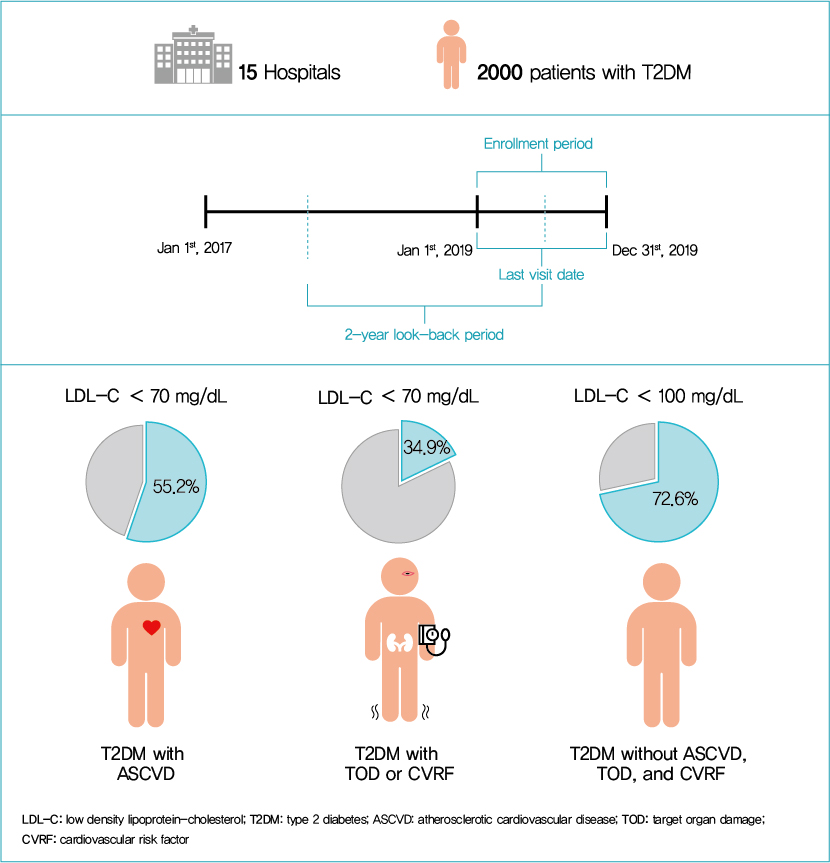
This retrospective cohort study collected electronic medical record data from patients with T2DM (≥20 years) managed by endocrinologists from 15 hospitals in Korea (January to December 2019). Patients were categorized according to guidelines to assess LDL-C target achievement. KDA (2019): Very High-I (atherosclerotic cardiovascular disease [ASCVD]) <70 mg/dL; Very High-II (target organ damage [TOD], or cardiovascular risk factors [CVRFs]) <70 mg/dL; high (others) <100 mg/dL. ESC/EAS (2019): Very High-I (ASCVD): <55 mg/dL; Very High-II (TOD or ≥3-CVRF) <55 mg/dL; high (diabetes ≥10 years without TOD plus any CVRF) <70 mg/dL; moderate (diabetes <10 years without CVRF) <100 mg/dL. ADA (2019): Very High-I (ASCVD); Very High-II (age ≥40+ TOD, or any CVRF), for high intensity statin or statin combined with ezetimibe.
Results
Among 2,000 T2DM patients (mean age 62.6 years; male 55.9%; mean glycosylated hemoglobin 7.2%) ASCVD prevalence was 24.7%. Of 1,455 (72.8%) patients treated with statins, 73.9% received monotherapy. According to KDA guidelines, LDL-C target achievement rates were 55.2% in Very High-I and 34.9% in Very High-II patients. With ESC/EAS guidelines, target attainment rates were 26.6% in Very High-I, 15.7% in Very High-II, and 25.9% in high risk patients. Based on ADA guidelines, most patients (78.9%) were very-high risk; however, only 15.5% received high-intensity statin or combination therapy.
Conclusion
According to current dyslipidemia management guidelines, LDL-C goal achievement remains suboptimal in Korean patients with T2DM. -
Citations
Citations to this article as recorded by- Risk factor control and cardiovascular events in patients with type 2 diabetes mellitus
Do Kyeong Song, Young Sun Hong, Yeon-Ah Sung, Hyejin Lee, Hidetaka Hamasaki
PLOS ONE.2024; 19(2): e0299035. CrossRef - Distinct effects of rosuvastatin and rosuvastatin/ezetimibe on senescence markers of CD8+ T cells in patients with type 2 diabetes mellitus: a randomized controlled trial
Sang-Hyeon Ju, Joung Youl Lim, Minchul Song, Ji Min Kim, Yea Eun Kang, Hyon-Seung Yi, Kyong Hye Joung, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
Frontiers in Endocrinology.2024;[Epub] CrossRef - Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Journal of Lipid and Atherosclerosis.2023; 12(1): 12. CrossRef - Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 1. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - Association between carotid atherosclerosis and presence of intracranial atherosclerosis using three-dimensional high-resolution vessel wall magnetic resonance imaging in asymptomatic patients with type 2 diabetes
Ji Eun Jun, You-Cheol Hwang, Kyu Jeong Ahn, Ho Yeon Chung, Geon-Ho Jahng, Soonchan Park, In-Kyung Jeong, Chang-Woo Ryu
Diabetes Research and Clinical Practice.2022; 191: 110067. CrossRef
- Risk factor control and cardiovascular events in patients with type 2 diabetes mellitus
- Comparison of Vildagliptin-Metformin and Glimepiride-Metformin Treatments in Type 2 Diabetic Patients
- Hyun Jeong Jeon, Tae Keun Oh
- Diabetes Metab J. 2011;35(5):529-535. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.529
- 65,535 View
- 96 Download
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The present study investigated the efficacy and safety of vildagliptin-metformin treatment compared to those of glimepiride-metformin treatment for type 2 diabetes.
Methods In a randomized, open-label, comparative study, 106 patients with type 2 diabetes were enrolled. The primary endpoint was a reduction in HbA1c from baseline and secondary endpoints included fasting plasma glucose (FPG) or 2-hour postprandial glucose (2h-PPG) reduction from baseline, as well as HbA1c responder rate and HbA1c reduction according to baseline HbA1c category.
Results Comparable HbA1c reduction was observed with a mean±standard deviation change from baseline to the 32-week endpoint of -0.94±1.15% in the vildagliptin group and -1.00±1.32% in the glimepiride group. A similar reduction in 2h-PPG (vildagliptin group 3.53±4.11 mmol/L vs. the glimepiride group 3.72±4.17 mmol/L) was demonstrated, and the decrements in FPG (vildagliptin group 1.54±2.41 mmol/L vs. glimepiride group 2.16±2.51 mmol/L) were not different between groups. The proportion of patients who achieved an HbA1c less than 7% at week 32 was 50.1% in the vildagliptin group and 56.0% in the glimepiride group. An average body weight gain of 2.53±1.21 kg in the glimepiride group was observed in contrast with the 0.23±0.69 kg weight gain noted in the vildagliptin group. A 10-fold lower incidence of hypoglycemia was demonstrated in the vildagliptin group, in addition to an absence of severe hypoglycemia.
Conclusion Vildagliptin-metformin treatment provided blood glucose control efficacy comparable to that of glimepiride-metformin treatment and resulted in better adverse event profiles with lower risks of hypoglycemia and weight gain.
-
Citations
Citations to this article as recorded by- A Randomized, Two-Treatments, Two-Periods, Crossover, Open label, Laboratory-Blind, Single Dose Bioequivalence Study between Vildagliptin/Metformin 50 mg/1000 mg Film Coated Tablets (Sensityn®) and Galvusmet® 50 mg/1000 mg Film Coated Tablets in healthy a
J. Shiekmydeen, T. Siddiqi, K. Chakraborty, S. Khalaf, M. Albarazi, I. Eqtefan, J. Sliva
European Pharmaceutical Journal.2023; 70(2): 1. CrossRef - Bioequivalence Studies of New Generic Formulations of Vildagliptin and Fixed-Drug Combination of Vildagliptin and Metformin Versus Respective Originator Products in Healthy Volunteers
Yvonne Schnaars, Sumedh Gaikwad, Ulrike Gottwald-Hostalek, Ulrike Klingberg, Hari Kiran Chary Vadla, Vamshi Ramana Prathap
Diabetes Therapy.2022; 13(6): 1215. CrossRef - Efficacy and safety of dorzagliatin for type 2 diabetes mellitus: A meta-analysis and trial sequential analysis
Yunfeng Yu, Xingyu Yang, Keke Tong, Shuang Yin, Gang Hu, Fei Zhang, Pengfei Jiang, Manli Zhou, Weixiong Jian
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - A Single-Center, Observational, Retrospective Cost-Effective Analysis of Treating Inadequately Controlled Type 2 Diabetes Mellitus by Addition of DPP4 Inhibitors Versus Intensified Treatment with Conventional Drugs
Akshata Kalyani, Sachin Kuchya, >Prashant Punekar
Journal of Pharmacology and Pharmacotherapeutics.2021; 12(3): 125. CrossRef - Comparison of safety and efficacy of glimepiride-metformin and vildagliptin- metformin treatment in newly diagnosed type 2 diabetic patients
Surendra Kumar
Indian Journal of Endocrinology and Metabolism.2021; 25(4): 326. CrossRef - Comparative clinical study evaluating the effect of adding Vildagliptin versus Glimepiride to ongoing Metformin therapy on diabetic patients with symptomatic coronary artery disease
Rehab Werida, Mahmoud Kabel, Gamal Omran, Ahmed Shokry, Tarek Mostafa
Diabetes Research and Clinical Practice.2020; 170: 108473. CrossRef - Efficacy of different antidiabetic drugs based on metformin in the treatment of type 2 diabetes mellitus: A network meta‐analysis involving eight eligible randomized‐controlled trials
Yan Peng, Shu‐Hong Chen, Xiao‐Nan Liu, Qing‐Yun Sun
Journal of Cellular Physiology.2019; 234(3): 2795. CrossRef - A safety and tolerability profile comparison between dipeptidyl peptidase-4 inhibitors and sulfonylureas in diabetic patients: A systematic review and meta-analysis
Daniela Farah, Graziella Malzoni Leme, Freddy Goldberg Eliaschewitz, Marcelo Cunio Machado Fonseca
Diabetes Research and Clinical Practice.2019; 149: 47. CrossRef - Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East‐Asian patients with type 2 diabetes in a multicentre, double‐blind, randomized, parallel‐arm, active comparator, phase III trial
Yu Hong Chen, Chien‐Ning Huang, Young Min Cho, Pengfei Li, Liqun Gu, Feng Wang, Jun Yang, Wei Qing Wang
Diabetes, Obesity and Metabolism.2018; 20(9): 2121. CrossRef - Cost effectiveness of vildagliptin versus glimepiride as add-on treatment to metformin for the treatment of diabetes mellitus type 2 patients in Greece
Hara Kousoulakou, Magdalini Hatzikou, Varvara Baroutsou, John Yfantopoulos
Cost Effectiveness and Resource Allocation.2017;[Epub] CrossRef - The efficacy and safety of adding either vildagliptin or glimepiride to ongoing metformin therapy in patients with type 2 diabetes mellitus
Gyuri Kim, Sewon Oh, Sang-Man Jin, Kyu Yeon Hur, Jae Hyeon Kim, Moon-Kyu Lee
Expert Opinion on Pharmacotherapy.2017; 18(12): 1179. CrossRef - Predictors of efficacy of GLP-1 agonists and DPP-4 inhibitors: A systematic review
Helene Bihan, Winda L. Ng, Dianna J. Magliano, Jonathan E. Shaw
Diabetes Research and Clinical Practice.2016; 121: 27. CrossRef - New Oral Diabetes Drugs are more effective than Older Agents: Real or a Fraud?
Udaya M Kabadi
Journal of Diabetes, Metabolic Disorders & Control.2016;[Epub] CrossRef - Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes
Eleni Bekiari, Chrysoula Rizava, Eleni Athanasiadou, Konstantinos Papatheodorou, Aris Liakos, Thomas Karagiannis, Maria Mainou, Maria Rika, Panagiota Boura, Apostolos Tsapas
Endocrine.2016; 52(3): 458. CrossRef - Sulfonylurea Glimepiride: A Proven Cost Effective, Safe and Reliable War Horse in Combating Hyperglycemia in Type 2 Diabetes
Udaya M. Kabadi
Journal of Diabetes Mellitus.2015; 05(04): 211. CrossRef - Glycemic effects of vildagliptin and metformin combination therapy in Indian patients with type 2 diabetes: An observational study (印度2型糖尿病患者使用维格列汀与二甲双胍联合治疗对血糖的影响:一项观察性研究)
Sanjay Chatterjee, Sudip Chatterjee
Journal of Diabetes.2014; 6(3): 237. CrossRef - Head‐to‐head comparison of dipeptidyl peptidase‐IV inhibitors and sulfonylureas – a meta‐analysis from randomized clinical trials
Yifei Zhang, Jie Hong, Jie Chi, Weiqiong Gu, Guang Ning, Weiqing Wang
Diabetes/Metabolism Research and Reviews.2014; 30(3): 241. CrossRef - Vildagliptin: A Review of Its Use in Type 2 Diabetes Mellitus
Gillian M. Keating
Drugs.2014; 74(5): 587. CrossRef - Vildagliptin compared to glimepiride on post-prandial lipemia and on insulin resistance in type 2 diabetic patients
Giuseppe Derosa, Aldo Bonaventura, Lucio Bianchi, Davide Romano, Elena Fogari, Angela D’Angelo, Pamela Maffioli
Metabolism.2014; 63(7): 957. CrossRef - The Placement of DPP-4 Inhibitors in Clinical Practice Recommendations for the Treatment of Types 2 Diabetes
Jaime A. Davidson
Endocrine Practice.2013; 19(6): 1050. CrossRef - Predictive Clinical Parameters and Glycemic Efficacy of Vildagliptin Treatment in Korean Subjects with Type 2 Diabetes
Jin-Sun Chang, Juyoung Shin, Hun-Sung Kim, Kyung-Hee Kim, Jeong-Ah Shin, Kun-Ho Yoon, Bong-Yun Cha, Ho-Young Son, Jae-Hyoung Cho
Diabetes & Metabolism Journal.2013; 37(1): 72. CrossRef - The Efficacy of Vildagliptin in Korean Patients with Type 2 Diabetes
Jun Sung Moon, Kyu Chang Won
Diabetes & Metabolism Journal.2013; 37(1): 36. CrossRef - Effect of Vildagliptin on Glucose and Insulin Concentrations During a 24-Hour Period in Type 2 Diabetes Patients with Different Ranges of Baseline Hemoglobin A1c Levels
Manuel González-Ortiz, María J. Sánchez-Peña, Luis J. González-Ortiz, José A. Robles-Cervantes, Yessica E. García-Ortega, Esteban A. Gómez-Gaitán, Karina G. Pérez-Rubio, Esperanza Martínez-Abundis
Diabetes Technology & Therapeutics.2013; 15(7): 564. CrossRef - Differential effects of vildagliptin and glimepiride on glucose fluctuations in patients with type 2 diabetes mellitus assessed using continuous glucose monitoring
Y. L. He, G. Foteinos, S. Neelakantham, D. Mattapalli, K. Kulmatycki, T. Forst, A. Taylor
Diabetes, Obesity and Metabolism.2013; 15(12): 1111. CrossRef - Combination therapy with metformin plus vildagliptin in type 2 diabetes mellitus
Elisa Guarino, Laura Nigi, Aurora Patti, Cecilia Fondelli, Francesco Dotta
Expert Opinion on Pharmacotherapy.2012; 13(9): 1377. CrossRef
- A Randomized, Two-Treatments, Two-Periods, Crossover, Open label, Laboratory-Blind, Single Dose Bioequivalence Study between Vildagliptin/Metformin 50 mg/1000 mg Film Coated Tablets (Sensityn®) and Galvusmet® 50 mg/1000 mg Film Coated Tablets in healthy a
- Association Study of the Peroxisome Proliferators-Activated Receptor gamma2 Pro12Ala Polymorphism with Diabetic Nephropathy.
- Kyu Ho Lee, Hee Seog Jeong, Khan Young Choi, Hyun Kim, Dal Sic Lee, Ji Young Kang, Hyun Jeong Jeon, Tae Keun Oh
- Korean Diabetes J. 2008;32(5):402-408. Published online October 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.5.402
- 1,910 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Peroxisome proliferators-activated receptor gamma (PPARgamma) is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors and known to play a role in regulating the expression of numerous genes involved in lipid metabolism, metabolic syndrome, inflammation, and atherosclerosis. The PPARgamma2 Pro12Ala polymorphism has recently been shown to be associated with diabetic nephropathy. In this study, we evaluated the relationship between PPARgamma2 Pro12Ala polymorphism and type 2 diabetic nephropathy whose duration of diabetes was over 10 years. METHODS: We conducted a case-control study, which enrolled 367 patients with type 2 diabetes. Genotyping of PPARgamma2 Pro12Ala polymorphism was performed using polymerase chain reaction followed by digestion with Hae III restriction enzyme. RESULTS: The genotype or allele frequencies of PPARgamma2 Pro12Ala polymorphism were not significantly different in diabetic patients with or without diabetic nephropathy. The genotype frequencies in terms of diabetic retinopathy and macrovascular complications such as coronary artery disease or stroke were not different either. Interestingly, nephropathy patients with Ala/Pro genotype showed lower C-peptide levels than those of Pro/Pro genotype. CONCLUSION: Our results suggest that PPARgamma2 Pro12Ala polymorphism is not associated with diabetic nephropathy in type 2 diabetic patients.
- Transforming Growth Factor-beta 1 Gene Polymorphisms According to Diabetic Nephropathy in Type 2 Diabetes.
- Hyun Jeong Jeon, Ok Hee Kim, Kil Ho, Soon Kil Kwon, Tae Keun Oh
- Korean Diabetes J. 2007;31(2):144-150. Published online March 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.2.144
- 1,416 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Transforming growth factor-beta is known to play a role in the interaction between metabolic and hemodynamic factors in mediating accumulation of extracellular matrix in the diabetic nephropathy. TGF-beta1 gene polymorphism was associated with circulating TGF-beta levels and influenced the pathogenesis of fibrotic diseases including diabetic nephropathy. In this study, we examined the relationship between TGF-beta1 gene codon 10 polymorphism and type 2 diabetic nephropathy with more than 10-year history of disease. METHODS: We conducted a case-control study, which enrolled 325 type 2 diabetes. A total of 176 patients with diabetic nephropathy were compared with 149 patients without diabetic nephropathy. TGF-beta1 codon 10 genotyping was determined using polymerase chain reaction with sequence specific primers method. RESULTS: Distribution of TGF-beta1 codon 10 genotype in the patients either with nephropathy or without nephropathy is confined to Hardy-Weinberg equilibrium. The patients with nephropathy have higher frequency of TGF-beta1 GA/GG genotypes than the patients without nephropathy [GA/GG:AA = 119 (67.6%) : 57 (32.4%) vs. 80 (53.7%) : 69 (46.3%), P < 0.05]. Among patients with diabetic nephropathy, those with TGF-beta1 GA/GG genotypes had higher serum levels of total cholesterol and LDL-cholesterol. CONCLUSION: Our results suggest that TGF-beta1 gene codon 10 polymorphism may contribute to the type 2 diabetic nephropathy.

 KDA
KDA
 First
First Prev
Prev





